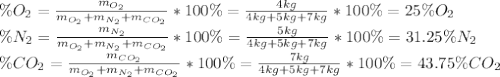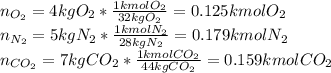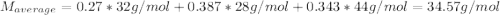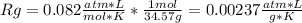Chemistry, 12.03.2020 00:29 damiangibson2
A gas mixture consists of 4 kg of O2, 5 kg of N2, and 7 kg of CO2. Determine (a) the mass fraction of each component, (b) the mole fraction of each component, and (c) the average molar mass and gas constant of the mixture.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
A gas mixture consists of 4 kg of O2, 5 kg of N2, and 7 kg of CO2. Determine (a) the mass fraction o...
Questions


English, 21.05.2021 15:20

SAT, 21.05.2021 15:20


English, 21.05.2021 15:20

Mathematics, 21.05.2021 15:20

Mathematics, 21.05.2021 15:20

Geography, 21.05.2021 15:20


Mathematics, 21.05.2021 15:20

History, 21.05.2021 15:20



English, 21.05.2021 15:20

History, 21.05.2021 15:20


English, 21.05.2021 15:20

Mathematics, 21.05.2021 15:20

Mathematics, 21.05.2021 15:20

English, 21.05.2021 15:20