The law of conservation of mass states that in a chemical reaction the mass of the reactants
eq...

Chemistry, 12.03.2020 00:31 GxthGrl6612
The law of conservation of mass states that in a chemical reaction the mass of the reactants
equals the mass of the products. Based on this information, what mass of hydrogen (H2)
was produced in this reaction?
A. 2 g
B. 4 g
C. 72 g
D. 144 g
please leave an explanation
thank you!
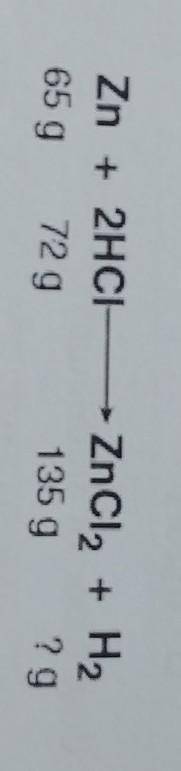

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
Questions


Computers and Technology, 01.12.2020 19:50

Biology, 01.12.2020 19:50

History, 01.12.2020 19:50






Spanish, 01.12.2020 19:50

History, 01.12.2020 19:50

Chemistry, 01.12.2020 19:50




Biology, 01.12.2020 19:50



Social Studies, 01.12.2020 19:50

Mathematics, 01.12.2020 19:50



