
Chemistry, 12.03.2020 01:06 bluenblonderw
A solution of I2was standardized with ascorbic acid. Using a 0.1000-g sample of pure ascorbic acid, 25.32 mL of I2 Are required to reach the starch end point. (a)What is the molarity of the iodine solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
A solution of I2was standardized with ascorbic acid. Using a 0.1000-g sample of pure ascorbic acid,...
Questions

Physics, 19.08.2021 20:50

Mathematics, 19.08.2021 20:50



World Languages, 19.08.2021 20:50

History, 19.08.2021 20:50

English, 19.08.2021 20:50




Mathematics, 19.08.2021 20:50




Social Studies, 19.08.2021 20:50

Social Studies, 19.08.2021 20:50


Chemistry, 19.08.2021 20:50

Mathematics, 19.08.2021 20:50

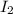 → dehydroascorbic acid +
→ dehydroascorbic acid + 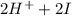
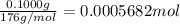
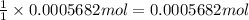 of
of ![[I_2]=\frac{0.0005682 mol}{0.02532 L}=0.02244 M](/tpl/images/0543/9758/3370c.png)



