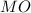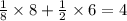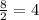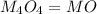Chemistry, 12.03.2020 01:46 Tcareyoliver
A solid metal oxide crystallizes in a cubic unit cell. In the unit cell there are metal (M) ions on every corner and in the center of every face. Oxide ions (O) are found in half of the tetrahedral holes. What is the empirical formula of this metal oxide?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1


Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
A solid metal oxide crystallizes in a cubic unit cell. In the unit cell there are metal (M) ions on...
Questions

Biology, 01.09.2019 01:10

Spanish, 01.09.2019 01:10



Mathematics, 01.09.2019 01:10

Computers and Technology, 01.09.2019 01:10

Health, 01.09.2019 01:10


Mathematics, 01.09.2019 01:10


Chemistry, 01.09.2019 01:10


Mathematics, 01.09.2019 01:20

Chemistry, 01.09.2019 01:20




Mathematics, 01.09.2019 01:20