
Chemistry, 12.03.2020 01:53 adrian08022
A buffered solution containing dissolved aniline, C 6 H 5 NH 2 , and aniline hydrochloride, C 6 H 5 NH 3 Cl , has a pH of 5.34 . A. Determine the concentration of C 6 H 5 NH 3 in the solution if the concentration of C 6 H 5 NH 2 is 0.245 M. The p K b of aniline is 9.13.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 23.06.2019 09:00
Individuals within populations exhibit some diversity. as a result of possessing slightly different traits, some individuals are better able to survive and reproduce than others. if these individuals changes in the characteristics of the population may occur over time. the cumulative change in these characteristics is known as
Answers: 3
You know the right answer?
A buffered solution containing dissolved aniline, C 6 H 5 NH 2 , and aniline hydrochloride, C 6 H 5...
Questions




English, 10.03.2020 19:08





Mathematics, 10.03.2020 19:08


Mathematics, 10.03.2020 19:08



Biology, 10.03.2020 19:08




Mathematics, 10.03.2020 19:08

Mathematics, 10.03.2020 19:08

English, 10.03.2020 19:08

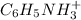 is, 0.0830 M
is, 0.0830 M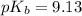
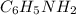 = 0.245 M
= 0.245 M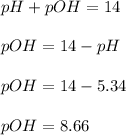
![pOH=pK_b+\log \frac{[Salt]}{[Base]}](/tpl/images/0544/0242/ac570.png)
![pOH=pK_b+\log \frac{[C_6H_5NH_3^+]}{[C_6H_5NH_2]}](/tpl/images/0544/0242/af8e2.png)
![8.66=9.13+\log (\frac{[C_6H_5NH_3^+]}{0.245})](/tpl/images/0544/0242/99488.png)
![[C_6H_5NH_3^+]=0.0830M](/tpl/images/0544/0242/f1802.png)


