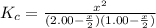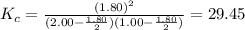Chemistry, 12.03.2020 02:05 martinbricein10
2.00 mol of H2(g) and 1.00 mol of I2(g) are placed in a 1.00 L container, and they react to form HI(g). At equilibrium, it is found that 1.80 moles of HI(g) are present in the container. Calculate K for the reaction: H2(g) + I2(g) ⇄ 2 HI(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3


Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1
You know the right answer?
2.00 mol of H2(g) and 1.00 mol of I2(g) are placed in a 1.00 L container, and they react to form HI(...
Questions






Chemistry, 06.06.2020 03:57




English, 06.06.2020 03:57

English, 06.06.2020 03:57




Mathematics, 06.06.2020 03:57


Mathematics, 06.06.2020 03:57

Physics, 06.06.2020 03:57


Mathematics, 06.06.2020 03:57

![[H_2]= \frac{2.00 mol}{1.00 L}=2.00 M](/tpl/images/0544/0690/a78aa.png)
![[I_2]= \frac{I.00 mol}{1.00 L}=1.00 M](/tpl/images/0544/0690/94c40.png)
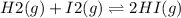
![[HI]=\frac{1.80 mol}{1.00L} = 1.80M= x](/tpl/images/0544/0690/bcad7.png)
![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0544/0690/62646.png)