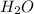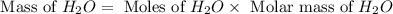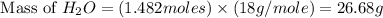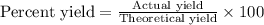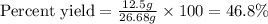Chemistry, 12.03.2020 03:51 brutalgitaffe
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . If of water is produced from the reaction of of sulfuric acid and of sodium hydroxide, calculate the percent yield of water. Round your answer to significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2


Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liqui...
Questions









History, 04.04.2020 04:39





Mathematics, 04.04.2020 04:39


English, 04.04.2020 04:39


History, 04.04.2020 04:39


Mathematics, 04.04.2020 04:39

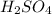 = 72.6 g
= 72.6 g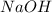 = 77.0 g
= 77.0 g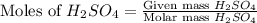
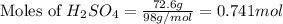
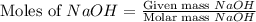
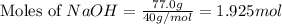
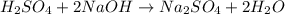
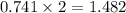 moles of
moles of