
Chemistry, 12.03.2020 04:10 dulaneystrode
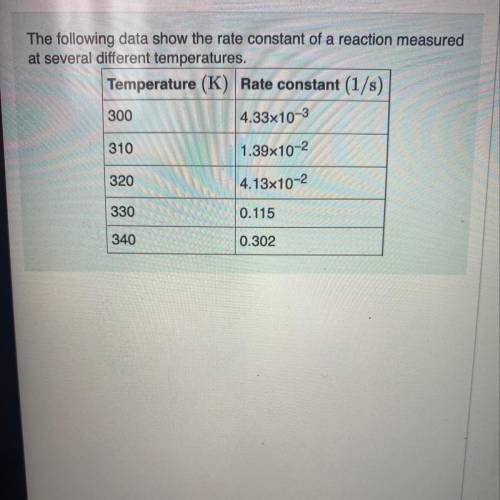
The following data show the rate constant of a reaction measured at several different temperatures.
Use an Arrhenius plot to determine the activation barrier (Ea) for the reaction.
Use an Arrhenius plot to determine the frequency (A) for the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3


Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
The following data show the rate constant of a reaction measured at several different temperatures.<...
Questions

Mathematics, 27.05.2021 01:20

History, 27.05.2021 01:20

History, 27.05.2021 01:20

History, 27.05.2021 01:20

English, 27.05.2021 01:20








History, 27.05.2021 01:20

Mathematics, 27.05.2021 01:20

English, 27.05.2021 01:20



Mathematics, 27.05.2021 01:20

Health, 27.05.2021 01:20

Mathematics, 27.05.2021 01:20



