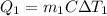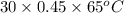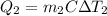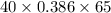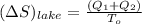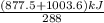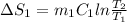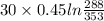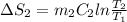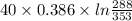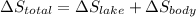Chemistry, 12.03.2020 05:14 Jwhite8602
A 30-kg iron block and a 40-kg copper block, both initially at 808C, are dropped into a large lake at 158C. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Determine the total entropy change for this process.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1


You know the right answer?
A 30-kg iron block and a 40-kg copper block, both initially at 808C, are dropped into a large lake a...
Questions









Computers and Technology, 02.10.2019 01:00

Computers and Technology, 02.10.2019 01:00



Mathematics, 02.10.2019 01:00



History, 02.10.2019 01:00



Business, 02.10.2019 01:00

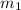 = 30 kg,
= 30 kg, 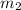 = 40 kg
= 40 kg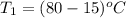 =
= 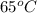
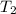 =
=