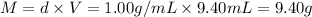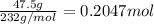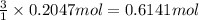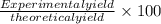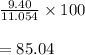Chemistry, 12.03.2020 05:32 kfcnkfnmnfk9513
What is the percent yield of a reaction in which 47.5 g tungsten (VI) oxide (WO3) reacts with excess hydrogen gas to produce metallic tungsten and 9.40 mL water (d = 1.00 g/mL)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
What is the percent yield of a reaction in which 47.5 g tungsten (VI) oxide (WO3) reacts with excess...
Questions


History, 13.12.2019 08:31

Physics, 13.12.2019 08:31

Social Studies, 13.12.2019 08:31


Mathematics, 13.12.2019 08:31

Social Studies, 13.12.2019 08:31

Mathematics, 13.12.2019 08:31

Social Studies, 13.12.2019 08:31


Social Studies, 13.12.2019 08:31


Social Studies, 13.12.2019 08:31


Mathematics, 13.12.2019 08:31

Mathematics, 13.12.2019 08:31

Mathematics, 13.12.2019 08:31



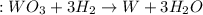
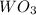 = 47.5g
= 47.5g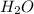 = 18 g/mole
= 18 g/mole