
Chemistry, 12.03.2020 05:41 Rachelmontes1
A 25-kg iron block initially at 350 oC is quenched in an insulated tank that contains 100 kg of water at 18 oC. Assuming the water that vaporizes during the process condenses back in the tank, determine the total entropy change during this process.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
A 25-kg iron block initially at 350 oC is quenched in an insulated tank that contains 100 kg of wate...
Questions






Mathematics, 04.12.2020 22:00



Chemistry, 04.12.2020 22:00

Mathematics, 04.12.2020 22:00

Social Studies, 04.12.2020 22:00


Mathematics, 04.12.2020 22:00

Geography, 04.12.2020 22:00

History, 04.12.2020 22:00

Social Studies, 04.12.2020 22:00

Mathematics, 04.12.2020 22:00


Mathematics, 04.12.2020 22:00

Mathematics, 04.12.2020 22:00

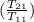 = 25 kg×0.444 J/g·°C㏑
= 25 kg×0.444 J/g·°C㏑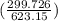 = -8124.296 J/K = -8.124 kJ/K
= -8124.296 J/K = -8.124 kJ/K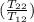 = 100 kg×4.186 J/g·°C㏑
= 100 kg×4.186 J/g·°C㏑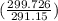 = 12152.01 J/K = 12.15201 kJ/K
= 12152.01 J/K = 12.15201 kJ/K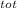 = ΔS₁ + ΔS₂ = -8.124 kJ/K + 12.15201 kJ/K = 4.03 kJ/K.
= ΔS₁ + ΔS₂ = -8.124 kJ/K + 12.15201 kJ/K = 4.03 kJ/K.

