Chemistry, 12.03.2020 16:55 sfigel3160
A reaction was performed in which 3.6 g 3.6 g of benzoic acid was reacted with excess methanol to make 1.3 g 1.3 g of methyl benzoate. Calculate the theoretical yield and percent yield for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
A reaction was performed in which 3.6 g 3.6 g of benzoic acid was reacted with excess methanol to ma...
Questions


Mathematics, 07.09.2021 01:30



Biology, 07.09.2021 01:30


Mathematics, 07.09.2021 01:30


English, 07.09.2021 01:40

Biology, 07.09.2021 01:40

Mathematics, 07.09.2021 01:40

Mathematics, 07.09.2021 01:40

History, 07.09.2021 01:40

Chemistry, 07.09.2021 01:40

Arts, 07.09.2021 01:40

Mathematics, 07.09.2021 01:40


Mathematics, 07.09.2021 01:40

Mathematics, 07.09.2021 01:40

Mathematics, 07.09.2021 01:40

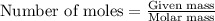 .....(1)
.....(1)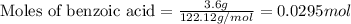
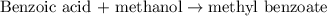
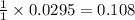 moles of methyl benzoate
moles of methyl benzoate