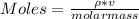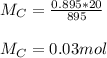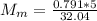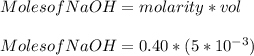You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of moles of vegetable oil, methanol, and NaOH that are initially present in the sample. Assume the density of vegetable oil is 0.895 g/mL and the molar mass is 895 g/mol. Look up the density and molar mass of any other compounds as needed.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3


Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1

You know the right answer?
You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of mol...
Questions


Geography, 16.07.2019 07:30


English, 16.07.2019 07:30


Mathematics, 16.07.2019 07:30

Biology, 16.07.2019 07:30



Mathematics, 16.07.2019 07:30

Mathematics, 16.07.2019 07:30


English, 16.07.2019 07:30

Mathematics, 16.07.2019 07:30



History, 16.07.2019 07:30

Mathematics, 16.07.2019 07:30