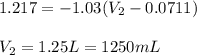Chemistry, 12.03.2020 17:32 jadenriley8129
A sample of gas occupies a volume of 71.1 mL . As it expands, it does 123.3 J of work on its surroundings at a constant pressure of 783 Torr . What is the final volume of the gas

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 10:30
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2

Chemistry, 23.06.2019 10:30
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1

Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1
You know the right answer?
A sample of gas occupies a volume of 71.1 mL . As it expands, it does 123.3 J of work on its surroun...
Questions





Social Studies, 12.04.2021 16:40





History, 12.04.2021 16:40


History, 12.04.2021 16:40



Mathematics, 12.04.2021 16:40

Mathematics, 12.04.2021 16:40


Health, 12.04.2021 16:40

Mathematics, 12.04.2021 16:40

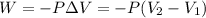
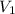 = initial volume = 71.1 mL = 0.0711 L (Conversion factor: 1 L = 1000 mL)
= initial volume = 71.1 mL = 0.0711 L (Conversion factor: 1 L = 1000 mL)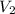 = final volume = ?
= final volume = ?