Chemistry, 12.03.2020 17:25 aubreymoore4553
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressure depression of 0.734 mmHg at 298 ∘C? (The vapor pressure of water at 298 K is 23.76 mmHg.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressur...
Questions





English, 19.08.2019 18:30



Computers and Technology, 19.08.2019 18:30

Mathematics, 19.08.2019 18:30

Mathematics, 19.08.2019 18:30

History, 19.08.2019 18:30

Mathematics, 19.08.2019 18:30


Mathematics, 19.08.2019 18:30

English, 19.08.2019 18:30

Mathematics, 19.08.2019 18:30

Social Studies, 19.08.2019 18:30


Mathematics, 19.08.2019 18:30

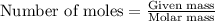
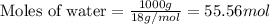
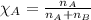
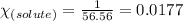
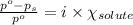
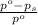 = relative lowering in vapor pressure = 0.734 mmHg
= relative lowering in vapor pressure = 0.734 mmHg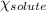 = mole fraction of solute = 0.0177
= mole fraction of solute = 0.0177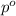 = vapor pressure of pure water = 23.76 torr
= vapor pressure of pure water = 23.76 torr