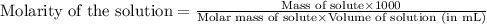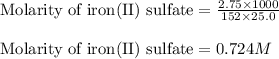Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2


Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
An aqueous solution of iron(II) sulfate (FeSO4) is prepared by dissolving 2.75 g in sufficient deion...
Questions




Mathematics, 31.01.2020 18:05



History, 31.01.2020 18:05




Mathematics, 31.01.2020 18:05

Mathematics, 31.01.2020 18:05



Mathematics, 31.01.2020 18:06

Mathematics, 31.01.2020 18:06