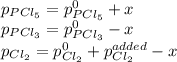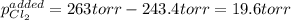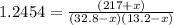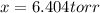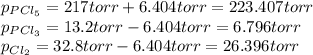Chemistry, 12.03.2020 21:01 jsmith4184
An equilibrium mixture of PCl 5 ( g ) , PCl 3 ( g ) , and Cl 2 ( g ) has partial pressures of 217.0 Torr, 13.2 Torr, and 13.2 Torr, respectively. A quantity of Cl 2 ( g ) is injected into the mixture, and the total pressure jumps to 263.0 Torr at the moment of mixing. The system then re-equilibrates. The chemical equation for this reaction is PCl 3 ( g ) + Cl 2 ( g ) − ⇀ ↽ − PCl 5 ( g ) Calculate the new partial pressures, P , after equilibrium is reestablished.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
You know the right answer?
An equilibrium mixture of PCl 5 ( g ) , PCl 3 ( g ) , and Cl 2 ( g ) has partial pressures of 217.0...
Questions


English, 15.10.2020 21:01


Spanish, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01


Mathematics, 15.10.2020 21:01

English, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01

Computers and Technology, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01

History, 15.10.2020 21:01


Computers and Technology, 15.10.2020 21:01


French, 15.10.2020 21:01

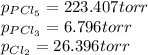
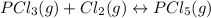
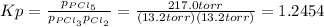
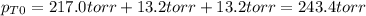
 owing to the chlorine's addition, turn out:
owing to the chlorine's addition, turn out: