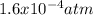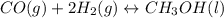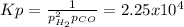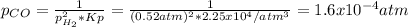Chemistry, 12.03.2020 22:09 demetriascott20
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium pressure of CO. CO(g) + 2 H2(g) CH3OH(l) Kp = 2.25 × 104 P(H2)eq = 0.52 atm

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particula...
Questions


English, 15.01.2021 17:30

Biology, 15.01.2021 17:30


Mathematics, 15.01.2021 17:30

Chemistry, 15.01.2021 17:30

French, 15.01.2021 17:30

Mathematics, 15.01.2021 17:30

Mathematics, 15.01.2021 17:30


Mathematics, 15.01.2021 17:30

Mathematics, 15.01.2021 17:30


English, 15.01.2021 17:30


Mathematics, 15.01.2021 17:30

Mathematics, 15.01.2021 17:30


Social Studies, 15.01.2021 17:30