
Chemistry, 12.03.2020 21:43 YoungKukie24
Consider the reaction 2NO(g) 1 O2(g) ¡ 2NO2(g) Suppose that at a particular moment during the reaction nitric oxide (NO) is reacting at the rate of 0.066 M/s. (a) At what rate is NO2 being formed? (b) At what rate is molecular oxygen reacting?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
Consider the reaction 2NO(g) 1 O2(g) ¡ 2NO2(g) Suppose that at a particular moment during the reacti...
Questions


Mathematics, 26.07.2021 15:00

English, 26.07.2021 15:00

English, 26.07.2021 15:00

Mathematics, 26.07.2021 15:00


Mathematics, 26.07.2021 15:00



English, 26.07.2021 15:00

English, 26.07.2021 15:00


Mathematics, 26.07.2021 15:00


Computers and Technology, 26.07.2021 15:00


Computers and Technology, 26.07.2021 15:00

Mathematics, 26.07.2021 15:00


English, 26.07.2021 15:00

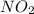 is formed is 0.066 M/sb) The rate at which molecular oxygen
is formed is 0.066 M/sb) The rate at which molecular oxygen 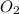 is reacting is 0.033 M/s
is reacting is 0.033 M/s
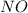 =
= ![-\frac{1d[NO]}{2dt}](/tpl/images/0545/5001/bf732.png) = 0.066 M/s
= 0.066 M/s![-\frac{1d[O_2]}{dt}](/tpl/images/0545/5001/1aba6.png)
![\frac{1d[NO_2]}{2dt}](/tpl/images/0545/5001/28990.png)
![-\frac{d[NO_2]}{2dt}=\frac{1d[NO]}{2dt}](/tpl/images/0545/5001/aed06.png)
![\frac{1d[NO_2]}{dt}=\frac{2}{2}\times 0.066M/s=0.066M/s](/tpl/images/0545/5001/e7a11.png)
![-\frac{1d[O_2]}{dt}=\frac{d[NO]}{2dt}](/tpl/images/0545/5001/c27b8.png)
![-\frac{1d[O_2]}{dt}=\frac{1}{2}\times 0.066M/s=0.033M/s](/tpl/images/0545/5001/6e7f3.png)


