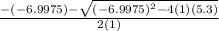Chemistry, 12.03.2020 21:40 daniellaZemira
Suppose a 250.0 mL flask is filled with 1.3 mol of I2 and 1.0 mol of HI. The following reaction becomes possible:
H2 (g) +I2 (g) ⇆ 2HI (g)
The equilibrium constant for this reaction is 0.983 at the temperature of the flask.
Calculate the equilibrium molarity of HI. Round your answer to two decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3


Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
Suppose a 250.0 mL flask is filled with 1.3 mol of I2 and 1.0 mol of HI. The following reaction beco...
Questions


History, 13.07.2020 17:01


Mathematics, 13.07.2020 17:01



Mathematics, 13.07.2020 17:01

Mathematics, 13.07.2020 17:01

History, 13.07.2020 17:01


Mathematics, 13.07.2020 17:01

Mathematics, 13.07.2020 17:01

Mathematics, 13.07.2020 17:01

History, 13.07.2020 17:01

Chemistry, 13.07.2020 17:01

Mathematics, 13.07.2020 17:01

Mathematics, 13.07.2020 17:01


Mathematics, 13.07.2020 17:01

Mathematics, 13.07.2020 17:01

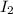 = 1.3 mol
= 1.3 mol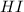 = 1.0 mole
= 1.0 mole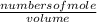
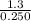
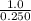
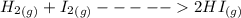
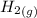 +
+ 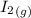 ----->
-----> 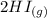
![K = \frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0545/4972/78f4e.png)
![K = \frac{[4-2x]^2}{[x][5.20+x]}](/tpl/images/0545/4972/b30d0.png) where K = 0.983
where K = 0.983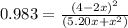
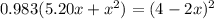
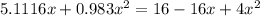
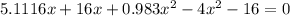
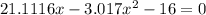
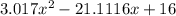
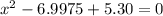
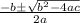
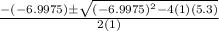
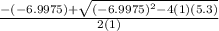 OR
OR