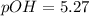Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1
You know the right answer?
If a 95.27 mL sample of acetic acid (HC2H3O2) is titrated to the equivalence point with 79.06 mL of...
Questions


Mathematics, 11.10.2021 07:50

Spanish, 11.10.2021 07:50

Mathematics, 11.10.2021 07:50

Computers and Technology, 11.10.2021 07:50


Biology, 11.10.2021 07:50

Mathematics, 11.10.2021 07:50

Mathematics, 11.10.2021 07:50

Mathematics, 11.10.2021 07:50

Mathematics, 11.10.2021 07:50


Business, 11.10.2021 07:50






Mathematics, 11.10.2021 08:00

Mathematics, 11.10.2021 08:00

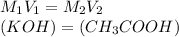
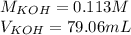
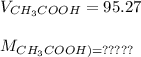
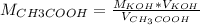
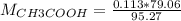
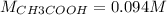
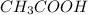 =
= 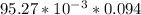
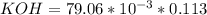

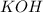 ----->
-----> 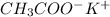
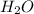
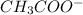 ion =
ion = 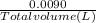
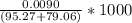
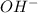
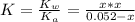
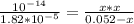
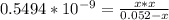
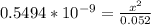

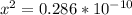
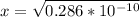
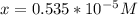
![[OH] = x =0.535*10^{-5}](/tpl/images/0545/6807/399d1.png)
![pOH = -log[OH^-]](/tpl/images/0545/6807/12649.png)
![pOH = -log[0.535*10^{-5}]](/tpl/images/0545/6807/26e4d.png)