Chemistry, 25.08.2019 17:50 fardinhaque6113
Suppose you have a calorimeter that contains 100.0 grams of water at an initial temperature of 25*c. a salt (2.19 g, 0.020 moles) is dissolved in the water, and the final temperature is 29*c. calculate the standard heat of solution (on a per mole basis). was the dissolution exothermic or endothermic?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Suppose you have a calorimeter that contains 100.0 grams of water at an initial temperature of 25*c....
Questions



Mathematics, 31.07.2019 20:10







Computers and Technology, 31.07.2019 20:20




Geography, 31.07.2019 20:20


History, 31.07.2019 20:20


English, 31.07.2019 20:20


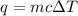
 = change in temperature =
= change in temperature = 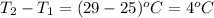
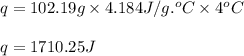
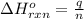
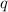 = amount of heat absorbed = 1710.25 J
= amount of heat absorbed = 1710.25 J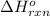 = standard enthalpy change of the reaction
= standard enthalpy change of the reaction