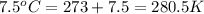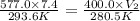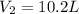A certain flexible weather balloon contains 7.4 L of helium gas. Initially, the balloon is in WP at 8500ft, where the temperature is 20.6oC and the barometric pressure is 577.0 torr. The balloon then is taken to the top of Pike’s Peak at an altitude of 14,100ft, where the pressure is 400 torr and the temperature is 7.5oC. What is the new volume of the balloon at the top of Pikes Peak?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
A certain flexible weather balloon contains 7.4 L of helium gas. Initially, the balloon is in WP at...
Questions


Mathematics, 16.10.2020 23:01

Social Studies, 16.10.2020 23:01



Mathematics, 16.10.2020 23:01

Mathematics, 16.10.2020 23:01

Chemistry, 16.10.2020 23:01

Geography, 16.10.2020 23:01



Health, 16.10.2020 23:01

Computers and Technology, 16.10.2020 23:01

Mathematics, 16.10.2020 23:01

Mathematics, 16.10.2020 23:01



Mathematics, 16.10.2020 23:01


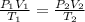
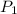 = initial pressure of gas = 577.0 torr
= initial pressure of gas = 577.0 torr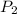 = final pressure of gas = 400 torr
= final pressure of gas = 400 torr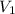 = initial volume of gas = 7.4 L
= initial volume of gas = 7.4 L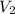 = final volume of gas = ?
= final volume of gas = ?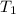 = initial temperature of gas =
= initial temperature of gas = 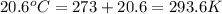
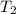 = final temperature of gas =
= final temperature of gas =