
Chemistry, 13.03.2020 00:58 ghwolf4p0m7x0
Avogadro's law predicts that the volume of a gas will increase with the addition of more moles of gas how does the kinetic theory explain this?
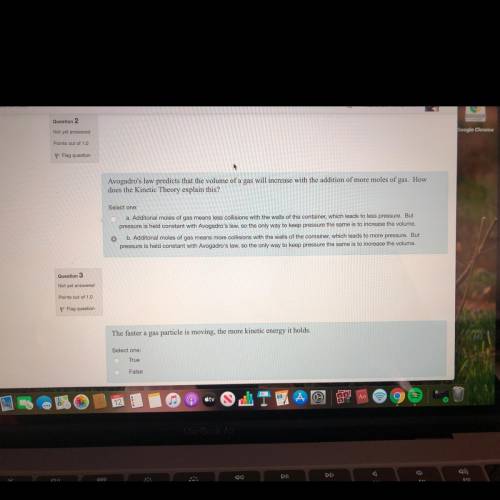

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
Avogadro's law predicts that the volume of a gas will increase with the addition of more moles of ga...
Questions

Spanish, 04.10.2019 19:50

Biology, 04.10.2019 19:50

English, 04.10.2019 20:00



Mathematics, 04.10.2019 20:00

Biology, 04.10.2019 20:00

Mathematics, 04.10.2019 20:00

History, 04.10.2019 20:00

Mathematics, 04.10.2019 20:00




Physics, 04.10.2019 20:00

Spanish, 04.10.2019 20:00




Mathematics, 04.10.2019 20:00

Physics, 04.10.2019 20:00





