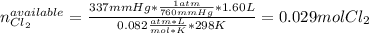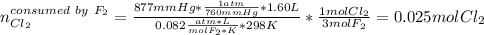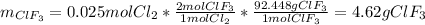Chemistry, 13.03.2020 01:35 tannercarr3441
Chlorine gas reacts with fluorine gas to form chlorine trifluoride. Cl2(g)+3F2(g)→2ClF3(g) A 1.60 L reaction vessel, initially at 298 K, contains chlorine gas at a partial pressure of 337 mmHg and fluorine gas at a partial pressure of 877 mmHg . Part A Identify the limiting reactant and determine the theoretical yield of ClF3 in grams.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1



Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
Chlorine gas reacts with fluorine gas to form chlorine trifluoride. Cl2(g)+3F2(g)→2ClF3(g) A 1.60 L...
Questions

Business, 11.01.2020 18:31


Mathematics, 11.01.2020 18:31

World Languages, 11.01.2020 18:31

Mathematics, 11.01.2020 18:31


History, 11.01.2020 18:31

English, 11.01.2020 18:31

Mathematics, 11.01.2020 18:31

Mathematics, 11.01.2020 18:31


Chemistry, 11.01.2020 18:31



History, 11.01.2020 18:31


Biology, 11.01.2020 18:31



Physics, 11.01.2020 18:31