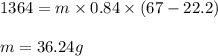Chemistry, 13.03.2020 02:28 alannaamarriee
A sample of sand initially at 22.2 °C absorbs 1364 J of heat. The final temperature of the sand is 67 °C. What is the mass (in g) of sand in the sample? The heat capacity of sand is 0.84

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3


Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
A sample of sand initially at 22.2 °C absorbs 1364 J of heat. The final temperature of the sand is 6...
Questions

English, 31.10.2019 17:31

Mathematics, 31.10.2019 17:31

Spanish, 31.10.2019 17:31

English, 31.10.2019 17:31

Health, 31.10.2019 17:31


Physics, 31.10.2019 17:31

History, 31.10.2019 17:31


Chemistry, 31.10.2019 17:31


Geography, 31.10.2019 17:31

Biology, 31.10.2019 17:31



Physics, 31.10.2019 17:31

Mathematics, 31.10.2019 17:31

English, 31.10.2019 17:31


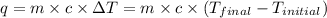
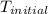 = initial temperature = 22.2°C
= initial temperature = 22.2°C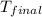 = final temperature = 67°C
= final temperature = 67°C