Chemistry, 13.03.2020 04:53 maevemboucher78
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.650 M, [B] = 1.35 M, and [C] = 0.300 M. The following reaction occurs and equilibrium is established: A+2B<->C
At equilibrium, [A] = 0.550 M and [B] = 0.400 M. Calculate the value of the equilibrium constant, Kc

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1


Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

You know the right answer?
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.650 M, [B] = 1.35...
Questions






Mathematics, 14.01.2020 23:31

Social Studies, 14.01.2020 23:31

English, 14.01.2020 23:31


Mathematics, 14.01.2020 23:31

Mathematics, 14.01.2020 23:31

Mathematics, 14.01.2020 23:31


Mathematics, 14.01.2020 23:31

Social Studies, 14.01.2020 23:31





Mathematics, 14.01.2020 23:31

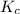 for given reaction is 0.465
for given reaction is 0.465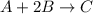
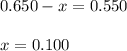
![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0546/2117/240ef.png)