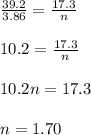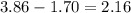Chemistry, 13.03.2020 04:28 bikerhomie
A container of acetylene gas (C2H2) holds 3.86mol of acetylene and has a volume of 39.2L. If there were a leak and the volume were to decrease to 17.3L at the same pressure and temperature, how many moles of acetylene would have been lost from the container?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

You know the right answer?
A container of acetylene gas (C2H2) holds 3.86mol of acetylene and has a volume of 39.2L. If there w...
Questions


Biology, 16.07.2019 03:30

Biology, 16.07.2019 03:30

Mathematics, 16.07.2019 03:30




Mathematics, 16.07.2019 03:30

Mathematics, 16.07.2019 03:30

Chemistry, 16.07.2019 03:30

History, 16.07.2019 03:30

Mathematics, 16.07.2019 03:30




Social Studies, 16.07.2019 03:30

Mathematics, 16.07.2019 03:30



Arts, 16.07.2019 03:30

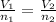 where V₁ is a volume, n₁ is a number in moles, V₂ is a second volume, and n₂ is its corresponding number in moles.
where V₁ is a volume, n₁ is a number in moles, V₂ is a second volume, and n₂ is its corresponding number in moles.