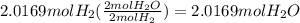27. For the reaction 2 H2 + O2 → 2 H20, how
many moles of H2O would you get from 2.0169
...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
Questions

Social Studies, 22.07.2019 13:00

Biology, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00


Mathematics, 22.07.2019 13:00






History, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00

Physics, 22.07.2019 13:00


Biology, 22.07.2019 13:00

Spanish, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00

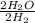 . The twos reduce, making this a one-to-one ratio.
. The twos reduce, making this a one-to-one ratio.