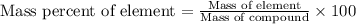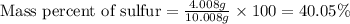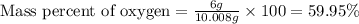Chemistry, 13.03.2020 17:28 anishivaturi123
When 4.008 g of sulfur is burned completely in abundant air, it combines with oxygen to form a single gaseous product with a total mass of 10.008 g. The mass percent of sulfur in the product is % (2 dec places). The mass percent of oxygen in the product is % (2 dec places).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 08:30
Which can be observed only in a microscopic view? a) structure of a muscle cell b) shape of a soybean plant c) foam insulation d) x-ray of a knee joint
Answers: 2

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1
You know the right answer?
When 4.008 g of sulfur is burned completely in abundant air, it combines with oxygen to form a singl...
Questions



Biology, 20.04.2020 16:57



History, 20.04.2020 16:57




Biology, 20.04.2020 16:58

Mathematics, 20.04.2020 16:58



Mathematics, 20.04.2020 16:58

History, 20.04.2020 16:58