
Chemistry, 13.03.2020 18:07 elliswilliams6035
Consider the following reaction: Consider the reaction 2NO(g)+Br2(g)⇌2NOBr(g) ,Kp=28.4 at 298 K In a reaction mixture at equilibrium, the partial pressure of NO is 119 torr and that of Br2 is 151 torr . What is the partial pressure of NOBr in this mixture?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1


Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2
You know the right answer?
Consider the following reaction: Consider the reaction 2NO(g)+Br2(g)⇌2NOBr(g) ,Kp=28.4 at 298 K In a...
Questions





Biology, 04.02.2020 03:46

Mathematics, 04.02.2020 03:46

Mathematics, 04.02.2020 03:46







History, 04.02.2020 03:46

Mathematics, 04.02.2020 03:46


Biology, 04.02.2020 03:46


English, 04.02.2020 03:46

Chemistry, 04.02.2020 03:46

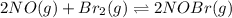
![K_{p}=\frac{[p_{NOBr}]^2}{[p_{NO}]^2\times [p_{Br_2}]^1}](/tpl/images/0546/6296/30bec.png)
![28.4=\frac{[p_{NOBr}]^2}{[(119)^2\times (151)^1}](/tpl/images/0546/6296/6ad3d.png)
![[p_{NOBr}]=7792](/tpl/images/0546/6296/2a41f.png) atm
atm

