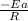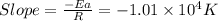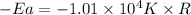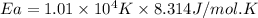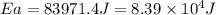Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1

Chemistry, 23.06.2019 12:30
Idid a lab for chemistry where we put nails in a copper (ii) chloride solution. 1. why did the reaction stop? which reactant was used up? how do you know? 2. describe what was happening to the atoms of iron and copper during the reaction. what is this type of reaction called? 3. what would happen to the ratio of copper to iron if you had placed more nails in the beaker? if you had let the reaction go for less time? 4. what is the accepted ratio of copper atoms to iron atoms in this reaction? account for differences between your experimental value and the accepted value. write the balanced equation for the reaction.
Answers: 2

Chemistry, 23.06.2019 20:30
Due tomorrow write the chemical equation that has the equilibrium constant expression [listed in photo]
Answers: 2
You know the right answer?
The rate consnt for a reaction is measured as a function of temperature. A plot of ln k versus 1/T i...
Questions

Social Studies, 13.08.2021 18:50

Social Studies, 13.08.2021 18:50


Mathematics, 13.08.2021 18:50

Mathematics, 13.08.2021 18:50






Computers and Technology, 13.08.2021 18:50



English, 13.08.2021 18:50

Social Studies, 13.08.2021 18:50


Biology, 13.08.2021 18:50



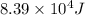
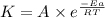
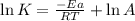
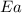 = activation energy for the reaction
= activation energy for the reaction