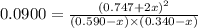Chemistry, 13.03.2020 19:56 donttrip10
A mixture of 0.590 M H 2 O , 0.340 M Cl 2 O , and 0.747 M HClO are enclosed in a vessel at 25 ° C . H 2 O ( g ) + Cl 2 O ( g ) − ⇀ ↽ − 2 HOCl ( g ) K c = 0.0900 at 25 ° C Calculate the equilibrium concentrations of each gas at 25 ° C .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1

Chemistry, 23.06.2019 18:30
You form water vapor by mixing oxygen and hydrogen at 730°c in a 5.4-liter container. this is the equation for the reaction: o2(g) + 2h2(g) → 2h2o(g). the partial pressure of oxygen before the reaction is 122.3 kilopascals, and there is excess hydrogen. how many moles of water are formed?
Answers: 3
You know the right answer?
A mixture of 0.590 M H 2 O , 0.340 M Cl 2 O , and 0.747 M HClO are enclosed in a vessel at 25 ° C ....
Questions





Mathematics, 24.07.2019 10:00

Biology, 24.07.2019 10:00







English, 24.07.2019 10:00




History, 24.07.2019 10:00



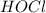 ,
, 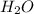 and
and 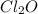 at equilibrium is, 0.215 M, 0.856 M and 0.606 M respectively.
at equilibrium is, 0.215 M, 0.856 M and 0.606 M respectively.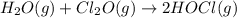
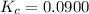
![K_c=\frac{[HOCl]^2}{[H_2O][Cl_2O]}](/tpl/images/0546/8405/da783.png)