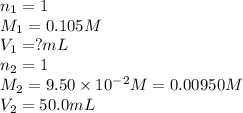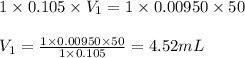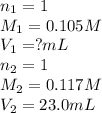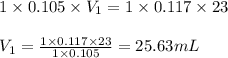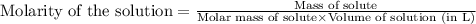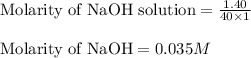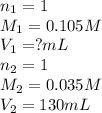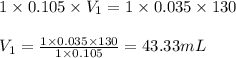Chemistry, 13.03.2020 22:21 WritingStar1313
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equivalence point?
a. 50.0 mL of 9.5010?2 M NaOH
b. 23.0 mL of 0.117 M NH3
c. 130 mL of a solution that contains 1.40 g of NaOH per liter

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

You know the right answer?
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equ...
Questions

Mathematics, 16.07.2019 03:30



Physics, 16.07.2019 03:30






History, 16.07.2019 03:30

English, 16.07.2019 03:30



Mathematics, 16.07.2019 03:30



Mathematics, 16.07.2019 03:30


Mathematics, 16.07.2019 03:30

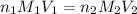
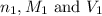 are the n-factor, molarity and volume of acid
are the n-factor, molarity and volume of acid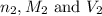 are the n-factor, molarity and volume of base
are the n-factor, molarity and volume of base