Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
If 1.785 g of ethanol (CHCHOH) is burned in a constant volume calorimeter causing a temperature incr...
Questions





Advanced Placement (AP), 19.05.2021 19:30




Mathematics, 19.05.2021 19:30


Physics, 19.05.2021 19:30



Mathematics, 19.05.2021 19:30

Mathematics, 19.05.2021 19:30


Mathematics, 19.05.2021 19:30

History, 19.05.2021 19:30

Mathematics, 19.05.2021 19:30

English, 19.05.2021 19:30

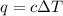
 = change in temperature = 4.32°C
= change in temperature = 4.32°C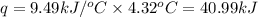
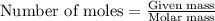
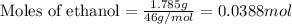
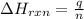
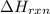 = enthalpy change of the reaction
= enthalpy change of the reaction