
Oxygen gas reacts with powdered aluminum according to the following reaction:
4Al(s)+3O2(g)→2Al2O3(s)
What volume of O2 gas, measured at 787 mmHg and 21 ∘C, is required to completely react with 55.0 g of Al?
Express the volume in liters to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
Oxygen gas reacts with powdered aluminum according to the following reaction:
4Al(s)+3O2...
4Al(s)+3O2...
Questions



Mathematics, 02.10.2020 08:01





Mathematics, 02.10.2020 08:01

History, 02.10.2020 08:01

Arts, 02.10.2020 08:01




History, 02.10.2020 08:01


Mathematics, 02.10.2020 08:01

History, 02.10.2020 08:01

Mathematics, 02.10.2020 08:01

Mathematics, 02.10.2020 08:01

Mathematics, 02.10.2020 08:01

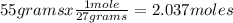
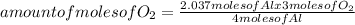
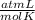 T= 21 C=294 K
T= 21 C=294 K

