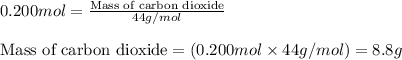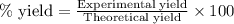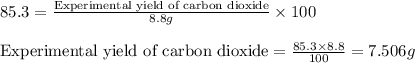Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
For the following reaction, 5.61 grams of carbon monoxide are mixed with excess water . Assume that...
Questions

Mathematics, 29.01.2021 21:20

Mathematics, 29.01.2021 21:20


SAT, 29.01.2021 21:20

Mathematics, 29.01.2021 21:20


Mathematics, 29.01.2021 21:20


Mathematics, 29.01.2021 21:20



Mathematics, 29.01.2021 21:20





History, 29.01.2021 21:20

Arts, 29.01.2021 21:20

English, 29.01.2021 21:20

Mathematics, 29.01.2021 21:20

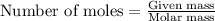 .....(1)
.....(1)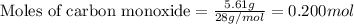
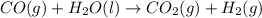
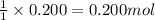 of carbon dioxide
of carbon dioxide