PLEASE ANSWER
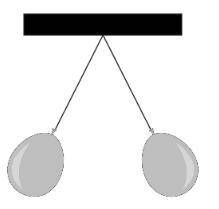
A student hangs two inflated balloons from 1-meter strings so that they to...

PLEASE ANSWER
A student hangs two inflated balloons from 1-meter strings so that they touch. The student then holds the balloons apart and rubs each balloon 5 times with animal fur. The student then releases the balloons. The balloons now hang a small distance apart from one another:
The next step in the student's procedure is to touch the balloons with a metal rod and allow them to settle, and then to rub them 10 times with animal fur. Which result should the student expect, and why?
A. The balloons will hang at a greater elevation, because they will have greater charges so the force of electrical repulsion between them will be greater.
B. The balloons will hang at a smaller elevation, because more thoroughly rubbing them will more completely remove their charges, reducing the electrical repulsion between them.
C. The balloons will hang at the same elevation, because electrical repulsion is an unvarying phenomenon which results from the existence of charge and not its quantity.
D. The balloons will hang straight down or more closely together, because a larger number of rubs with fur will give them opposite rather than like electrical charges.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 05:30
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
Questions

History, 26.04.2021 22:50

Physics, 26.04.2021 22:50

English, 26.04.2021 22:50

Social Studies, 26.04.2021 22:50

History, 26.04.2021 22:50



Mathematics, 26.04.2021 22:50

Chemistry, 26.04.2021 22:50

Mathematics, 26.04.2021 22:50



English, 26.04.2021 22:50

Computers and Technology, 26.04.2021 22:50

History, 26.04.2021 22:50



Mathematics, 26.04.2021 22:50

Mathematics, 26.04.2021 22:50



