
Chemistry, 14.03.2020 06:55 rosemarybooker
The following reaction is spontaneous as written when the components are in their standard states:
3 Zn(s) +2 Cr3+(aq) →3 Zn2+(aq) +2 Cr(s)
If the [Zn2+] is 4 molL−1, determine the value of [Cr3+] below which the reaction will be spontaneous in the opposite direction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

You know the right answer?
The following reaction is spontaneous as written when the components are in their standard states:
Questions


History, 10.03.2021 14:00



Biology, 10.03.2021 14:00



Mathematics, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00

English, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00

Social Studies, 10.03.2021 14:00



Chemistry, 10.03.2021 14:00

Biology, 10.03.2021 14:00

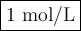
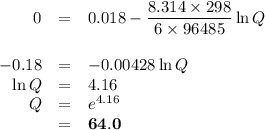
![\begin{array}{rcl}Q & = & \dfrac{\text{[Zn$^{2+}$]$^{3}$}}{\text{[Cr}^{3+}]^{2}}\\\\64.0 & = & \dfrac{4^{3}}{\text{[Cr}^{3+}]^{2}}\\\\\text{[Cr}^{3+}]^{2}& = & \dfrac{64}{64.0}\\\\& = & 1\\\text{[Cr}^{3+}] & = & \textbf{1 mol/L}\\\end{array}\\\text{[Cr$^{3+}$] must be less than $\large \boxed{\textbf{1 mol/L}}$ for the reaction to be spontaneous in the reverse}\\\text{direction.}](/tpl/images/0547/6685/5fcfe.png)


