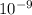Chemistry, 15.03.2020 01:53 harleycrider2251
Determine the [H3O+] in a 0.265 M HClO solution. The Ka of HClO is 2.9 × 10-8.
a. 1.3 × 10-6 M
b. 7.7 × 10-9 M
c. 1.1 × 10-10 M
d. 8.8 × 10-5 M
e. 4.9 × 10-4 M

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
Determine the [H3O+] in a 0.265 M HClO solution. The Ka of HClO is 2.9 × 10-8.
a. 1.3 ×...
a. 1.3 ×...
Questions


Mathematics, 15.04.2020 02:05






Mathematics, 15.04.2020 02:05





Mathematics, 15.04.2020 02:06




Mathematics, 15.04.2020 02:06

Mathematics, 15.04.2020 02:06

English, 15.04.2020 02:06

![Ka=\frac{[H_{3} O^{+}][ClO^{-}] }{[HClO]}](/tpl/images/0547/8898/f175d.png)
![2.9x10^{-8} =\frac{[x][x] }{0.265-x}](/tpl/images/0547/8898/9fe65.png)
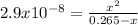
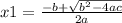 and
and 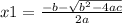
![\frac{[ClO-][H3O+]}{[HClO]}](/tpl/images/0547/8898/317ca.png)
![\frac{[x] [x]}{[0.265-x]}](/tpl/images/0547/8898/b1ba9.png)
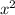 = 7.698 x
= 7.698 x