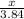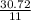Chemistry, 15.03.2020 02:28 poweradampower
The chemical reaction in this example is of environmental interest. Iron pyrite (Fesz) is often an impurity in
coal, and so burning this fuel in a power plant produces sulfur dioxide (SO), a major air pollutant.
Calculate the mass of sulfur dioxide (SO2) produced when 3.84 mol O2 is reacted with Fes, according to the
equation
4 FeSacs + 11 O.
- 2 Fe2O3(-) + S SO2(e)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2


Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

You know the right answer?
The chemical reaction in this example is of environmental interest. Iron pyrite (Fesz) is often an i...
Questions

History, 05.02.2020 00:53








Mathematics, 05.02.2020 00:53

History, 05.02.2020 00:53


Mathematics, 05.02.2020 00:53

Mathematics, 05.02.2020 00:53



Mathematics, 05.02.2020 00:53

Geography, 05.02.2020 00:53

History, 05.02.2020 00:53


Mathematics, 05.02.2020 00:53

 =
=