
Balance the following chemical equation by the half-reaction method in acidic conditions. Show your work. P4 + NO3–→ H2PO4– + NO(A) What is the oxidized element? What is the reduced element?
(B) Write balanced half reactions for the oxidation reaction:
(C) Write balanced half reactions for the reduction reaction:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

You know the right answer?
Balance the following chemical equation by the half-reaction method in acidic conditions. Show your...
Questions


History, 21.02.2020 00:28






Social Studies, 21.02.2020 00:28

Mathematics, 21.02.2020 00:28




Mathematics, 21.02.2020 00:28


Social Studies, 21.02.2020 00:28





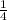 P4 + 4H2O ⇒ H2PO4- + 6H+ + 5e
P4 + 4H2O ⇒ H2PO4- + 6H+ + 5e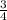 P4 + 5NO3- +2H+ + 2H2O⇒ 5NO+3H3PO4-
P4 + 5NO3- +2H+ + 2H2O⇒ 5NO+3H3PO4-

