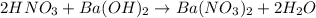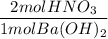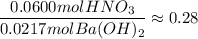Chemistry, 16.03.2020 06:22 kameronstebbins
If 10.0 mL of a .600 M of HNO3 reacts with 31.0 mL of .700M Ba(OH)2 solution, what is the molarity of Ba(OH)2 after the reaction is complete

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
If 10.0 mL of a .600 M of HNO3 reacts with 31.0 mL of .700M Ba(OH)2 solution, what is the molarity o...
Questions

Mathematics, 28.06.2019 09:30

Mathematics, 28.06.2019 09:30

History, 28.06.2019 09:30

Mathematics, 28.06.2019 09:30

Mathematics, 28.06.2019 09:30

Health, 28.06.2019 09:30

Chemistry, 28.06.2019 09:30

Biology, 28.06.2019 09:30

Mathematics, 28.06.2019 09:30

Biology, 28.06.2019 09:30



History, 28.06.2019 09:30

Mathematics, 28.06.2019 09:30

Chemistry, 28.06.2019 09:30


History, 28.06.2019 09:30


Mathematics, 28.06.2019 09:30

Mathematics, 28.06.2019 09:30