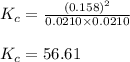Chemistry, 16.03.2020 16:29 jennemylesp19oy5
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture is heated to 425oC. At equilibrium, the concentration of I2is found to be 0.0210 M. Calculate Kcfor the following reaction at 425oC. H2(g) + I2(g) ⇄2 HI(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture i...
Questions

Mathematics, 15.03.2022 17:20

Mathematics, 15.03.2022 17:20


Mathematics, 15.03.2022 17:20



Spanish, 15.03.2022 17:20



Physics, 15.03.2022 17:30

Mathematics, 15.03.2022 17:30

Mathematics, 15.03.2022 17:40

Computers and Technology, 15.03.2022 17:40

History, 15.03.2022 17:40

Mathematics, 15.03.2022 17:40

Advanced Placement (AP), 15.03.2022 17:40

Mathematics, 15.03.2022 17:40

Business, 15.03.2022 17:40

Mathematics, 15.03.2022 17:40

Mathematics, 15.03.2022 17:40

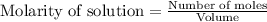
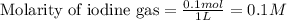
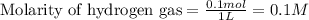
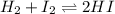
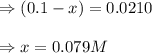
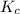 for above equation follows:
for above equation follows:![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0548/4996/62646.png)
![[HI]_{eq}=2x=(2\times 0.079)=0.158M](/tpl/images/0548/4996/cad3c.png)
![[H_2]_{eq}=(0.1-x)=(0.1-0.079)=0.0210M](/tpl/images/0548/4996/029c8.png)
![[I_2]_{eq}=0.0210M](/tpl/images/0548/4996/0be20.png)