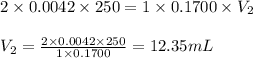Chemistry, 16.03.2020 16:37 DonovanBaily42
A chemist weighs out 0.0865 g of sulfurous acid, H2SO3, which is a diprotic acid into a 250. mL of volumetric flask and dilutes to the mark with distilled water. She plans to titrate the acid with 0.1700 M NaOH solution. Calculate the volume of NaOH solution in milliliters the student will need to add to reach the final equivalence point. (Molar mass of sulfurous acid = 82.079 g/moL)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
A chemist weighs out 0.0865 g of sulfurous acid, H2SO3, which is a diprotic acid into a 250. mL of v...
Questions


Social Studies, 13.10.2020 03:01


Mathematics, 13.10.2020 03:01


History, 13.10.2020 03:01

Geography, 13.10.2020 03:01

Mathematics, 13.10.2020 03:01

Chemistry, 13.10.2020 03:01

Mathematics, 13.10.2020 03:01

Health, 13.10.2020 03:01

Mathematics, 13.10.2020 03:01

Mathematics, 13.10.2020 03:01


Health, 13.10.2020 03:01





Health, 13.10.2020 03:01

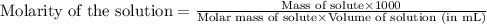


 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 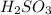
 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.