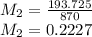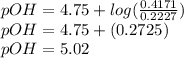Chemistry, 16.03.2020 16:59 dlewis9632
A buffer is made by combining 5.55 g of NH3(Kb= 1.8 X 10-5)with 4.78 g of HCl and diluting to a volume of 750.0 mL. a. What is the pH?b. What is the pH after adding 60.0 mL of 2.00 M HBr(aq)?c. What is the pH after adding 120.0 mL of 2.00 M HBr(aq)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3


Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
A buffer is made by combining 5.55 g of NH3(Kb= 1.8 X 10-5)with 4.78 g of HCl and diluting to a volu...
Questions


Mathematics, 16.06.2021 01:40





Computers and Technology, 16.06.2021 01:40


Computers and Technology, 16.06.2021 01:40








Social Studies, 16.06.2021 01:40



Biology, 16.06.2021 01:40

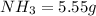
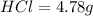
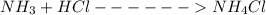
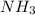 = 17 g/mol
= 17 g/mol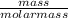
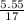
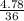
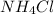
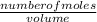
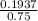
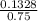
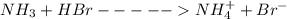
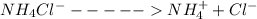
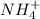 is present in both of the above reactions; then this a buffer solution which is demonstrated by using Henderson Hasslelbach equation.
is present in both of the above reactions; then this a buffer solution which is demonstrated by using Henderson Hasslelbach equation.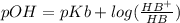
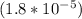
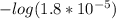
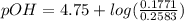
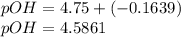
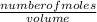
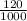
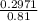
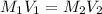 ; we have :
; we have :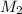 × 810
× 810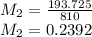
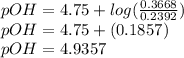
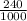
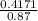
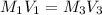 ; we have :
; we have :