
Chemistry, 16.03.2020 17:31 Savannahh8503
The formation constant* of [M(CN) 4 ]2− is 7.70 × 10 16 , where M is a generic metal. A 0.150 mole quantity of M(NO3)2 is added to a liter of 0.820 M NaCN solution. What is the concentration of M2+ ions at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1



Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1
You know the right answer?
The formation constant* of [M(CN) 4 ]2− is 7.70 × 10 16 , where M is a generic metal. A 0.150 mole q...
Questions

Mathematics, 11.11.2020 09:00


History, 11.11.2020 09:00

Business, 11.11.2020 09:00

Mathematics, 11.11.2020 09:00

Chemistry, 11.11.2020 09:00


English, 11.11.2020 09:00

History, 11.11.2020 09:00

History, 11.11.2020 09:00

Social Studies, 11.11.2020 09:00

History, 11.11.2020 09:00


Biology, 11.11.2020 09:00

Mathematics, 11.11.2020 09:00

Biology, 11.11.2020 09:00

Mathematics, 11.11.2020 09:00



![M(NO_3)_2+NaCN\leftrightarrow [M(CN)_4]^{-2}+NaNO_3](/tpl/images/0548/6085/c1450.png)
![M^{+2}+4CN^-\leftrightarrow [M(CN)_4]^{-2}](/tpl/images/0548/6085/1b38d.png)
![K_F=\frac{[[M(CN)_4]^{-2}]_{eq}}{[M^{+2}]_{eq}[CN^{-}]_{eq}^4}](/tpl/images/0548/6085/aa364.png)
![[M^{+2}]_0=0.150M;[CN^-]_0=0.820M](/tpl/images/0548/6085/52301.png)
 due to the reaction progress:
due to the reaction progress: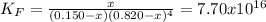
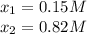
![[M^{+2}]_{eq}=0.15M-0.15M=0M](/tpl/images/0548/6085/e5b91.png)



