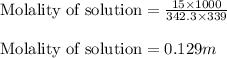Chemistry, 16.03.2020 18:07 angelthompson2018
A student dissolves 15.g of sucrose C12H22O11 in 300.mL of a solvent with a density of 1.13/gmL. The student notices that the volume of the solvent does not change when the sucrose dissolves in it. Calculate the molarity and molality of the student's solution. Be sure each of your answer entries has the correct number of significant digits

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
A student dissolves 15.g of sucrose C12H22O11 in 300.mL of a solvent with a density of 1.13/gmL. The...
Questions

Biology, 13.02.2022 07:40


English, 13.02.2022 07:40

Biology, 13.02.2022 07:40



Social Studies, 13.02.2022 07:40

Mathematics, 13.02.2022 07:40

Mathematics, 13.02.2022 07:40


History, 13.02.2022 07:40




Mathematics, 13.02.2022 07:40



Mathematics, 13.02.2022 07:40


Biology, 13.02.2022 07:40

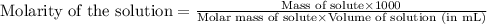
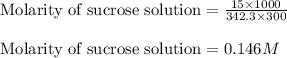
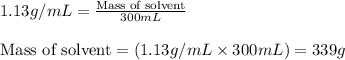
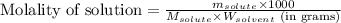
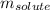 = Given mass of solute (sucrose) = 15 g
= Given mass of solute (sucrose) = 15 g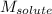 = Molar mass of solute (sucrose) = 342.3 g/mol
= Molar mass of solute (sucrose) = 342.3 g/mol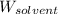 = Mass of solvent = 339 g
= Mass of solvent = 339 g