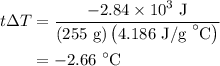Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2


Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
An 8.5g ice cube is placed into 255g of water. calculate the temperature change in the water upon th...
Questions

English, 02.07.2019 02:00

History, 02.07.2019 02:00

Mathematics, 02.07.2019 02:00

Mathematics, 02.07.2019 02:00

Mathematics, 02.07.2019 02:00


Mathematics, 02.07.2019 02:00

Mathematics, 02.07.2019 02:00


Mathematics, 02.07.2019 02:00










 .
.
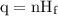 …… (1)
…… (1)
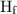 is latent heat of fusion.
is latent heat of fusion.
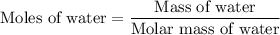 …… (2)
…… (2)
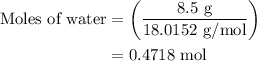
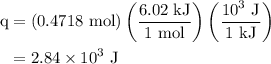
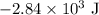 .
.
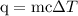 …… (3)
…… (3)
 is change in temperature.
is change in temperature.
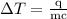 …… (4)
…… (4)
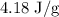 for c in equation (4).
for c in equation (4).