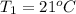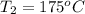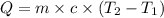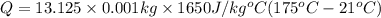Chemistry, 16.03.2020 18:16 deidaralove90
How much energy must be added to a bowl of 125popcorn kernels in order for them to reach a popping temperature of 175°C? Assume that their initial temperature is 21°C, that the specific heat capacity of popcorn is 1650 J/kg•°C, and that each kernel has a mass of 0.105 g.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1
You know the right answer?
How much energy must be added to a bowl of 125popcorn kernels in order for them to reach a popping t...
Questions


Mathematics, 19.11.2020 18:20

Chemistry, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20




Mathematics, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20


Mathematics, 19.11.2020 18:20



Biology, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20